E210 - Benzoic Acid
 Author: Alexander Bruni
Time for reading: ~1
minutes
Last Updated:
August 08, 2022
Author: Alexander Bruni
Time for reading: ~1
minutes
Last Updated:
August 08, 2022
CHAPTERS (Table Of Contents)
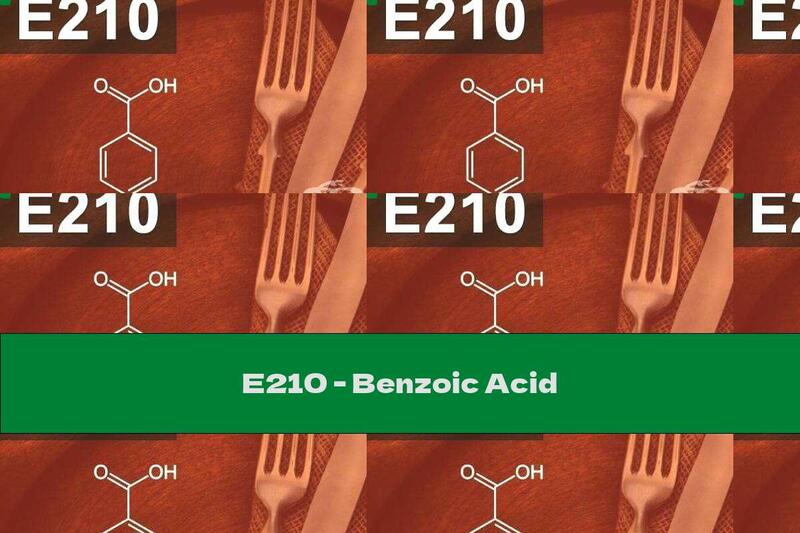
Characteristic: Benzoic acid is described as shiny leaves or white needles that melt at 122C. The acid is soluble: in 100 grams of water (at room temperature) dissolve 0.34 grams of benzene
Characteristic
Benzoic acid is described as shiny leaves or white needles that melt at 122 ° C. The acid is soluble: in 100 grams of water (at room temperature) dissolve 0.34 grams of benzoic acid, in fat - 1.2 mg of acid in 100 grams of oil and anhydrous ethanol.
From a chemical point of view, the acid refers to aromatic carboxylic acids. It is a natural substance found in some berries (blueberries). Benzoic acid is formed when microbial decomposition of hippuric acid takes place in fermented milk products. The antimicrobial action is based on the suppression of enzymes in microbial cells.
Large amounts of E 210 are obtained by oxidation of toluene.
Benzoic acid is not an expensive preservative, but has recently been replaced by others that are less toxic.
Use
E 210 is used in the food industry: in the production of beer, bread and bakery products, in confectionery, in fruit juices, margarine, marinated fish, pickles, in the dairy industry, in chewing gum, spices, ice cream, liqueurs and sweeteners.
Benzoic acid is used in cosmetics together with its salts and esters, in pharmacy - in the form of benzyl benzoate (up to 25%) in ointments against scabies. It is used for medicinal purposes as an antimicrobial and fungicidal agent. In many cough medicines, the substance acts as an antiseptic and has expectorant properties. It has been proven in the treatment of fungal diseases of the skin and sweating of the feet. This acid is important for the chemical industry because it is a major reagent for the synthesis of many chemicals.
Impact on humans
Benzoic acid is well absorbed by humans, interacts with protein compounds, forming hippuric acid and in this form is excreted by the kidneys.
There is evidence that benzoic acid and sodium benzoate interact with ascorbic acid (eg in soft drinks) to form benzene, which is carcinogenic. Therefore, it is better not to consume drinks that contain both benzoic acid and vitamin C.
In some countries, a maximum level of preservative in products is set = 5 ml / kg. Higher doses do not work well on the liver and kidneys.
Related Articles
- The Ultimate Guide to Lactic Acid in Nutrition: Benefits, Food Sources, and Recipes
- The Role of E210 in Nutrition: Benefits, Concerns, and Regulations
- The Role of Fatty Acids in Nutrition: Benefits, Sources, and Intake
- The Complete Guide to E952 (Cyclamic Acid) in Nutrition
- The Nutritional Benefits of Blubber: Omega-3 Fatty Acids and Vitamin D